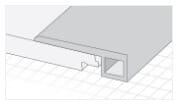
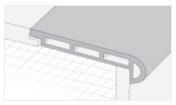
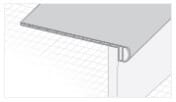
Accessoires
Accessoires de planchers
La qualité réside dans les détails. Les accessoires des planchers Cali Vinyl donneront à l’ensemble de votre projet un aspect élégant et professionnel.
Tous les accessoires sont disponibles sur demande et sont assortis à la couleur de votre plancher.
Installation et maintenance
Effectuez l’installation et la maintenance de votre plancher comme un PRO!
Nous avons rassemblé dans ces documents toute l’information nécessaire à la bonne conduite de votre projet pour assurer une installation tout à fait impeccable.
Vous avez des questions additionnelles? Faites-nous signe !
Guide Pré-Installation
Évitez le dérapage en suivant ces deux étapes les plus importantes, l’acclimatation et les joints d’expansion.
Guides d’Installation
Simples à suivre voici les instructions officielles d’installation des planchers Cali Bamboo pour installer en toute confiance et assurer la réussite de votre projet.
Entretien & Maintenance
Apprenez à maintenir votre plancher en parfaite condition pour des années à venir en suivant ces étapes simples et efficaces pour l’entretien de votre nouveau plancher.
FAQ
Q. Comment réagit le Vinyle Cali à l’égard des fuites et liquides?
R. Tous les planchers de Cali Vinyle sont 100% à l’épreuve de l’eau et sont conçus pour résister au trafic élevé et à l’humidité. Sa structure imperméable, incluant la membrane isolante intégrée de liège a été conçue pour rencontrer les exigences commerciales, les codes régissant les multi-étages résidentiels ainsi que les environnements pour les personnes âgées et les étudiants.
Q. Est-ce que vos planchers de bambou sont sécuritaires pour ma famille?
R. Lorsqu’il s’agit de qualité d’air intérieur, notre préoccupation première demeure le niveau de concentration des émissions de composés organiques volatiles (COV) . Seuls des techniques de fabrication et adhésifs de haute qualité sont utilisés dans le processus de fabrication pour assurer que tous les planchers soient libres de tout produit chimique toxique. Tous les planchers de Cali Bamboo sont testés par Benchmark International, un des laboratoires les plus reconnus pour la détection des composés organiques volatiles (COV) incluant le formaldéhyde. La plupart de nos planchers enregistrent des niveaux « non-détectables ». Même ceux affichant un niveau détectable sont tout de même 25 fois plus bas que les standards les plus rigoureux CARB (California Air Resources Board) Phase2 (0.05 PPM) et rencontrent au minimum la moitié de la concentration rencontrée dans l’air typiquement que nous respirons. (0.02PPM)
Tous nos planchers sont Ultra Bas en COV, certifiés FloorScore® , la plus haute reconnaissance en qualité de l’air de l’industrie. Un exemple de résultats obtenus pour nos vinyles est disponible dans la section des spécifications techniques de chacun des planchers.
Q. Est-ce que le plancher de Vinyle Cali sera approprié pour ma région ou mon climat?
R. Oui! Puisque les planchers de vinyle prennent de l’expansion et se contractent de façon minimale avec les changements de température et d’humidité. Ce plancher sera tout à fait approprié pour toutes les régions du Canada et des États-Unis. Assurez-vous cependant de bien suivre les directives d’installation de notre guide pour que votre plancher dure toute votre vie.
Q. Comment dois-je installer le plancher Cali Vinyle?
R. Vous pouvez trouver notre guide d’installation facile à suivre dans l’onglet « Installation et Maintenance » dans cette même section.
Q. Vendez-vous les accessoires en vinyle tels que les pièces pour escalier et moulures de transition?
R. Oui! Tous les planchers Cali Vinyle sont assortis d’accessoires présentant le même fini et couleur pour donner à l’ensemble de votre projet un aspect professionnel. Les accessoires disponibles sont les marches d’escalier et les contremarches, les nez-de-paliers, réductions, transitions, quarts-de-ronds, seuils et plinthes. Voir l’onglet « Accessoires » dans cette même section pour plus de détails.
Q. Est-ce que les planchers Cali Vinyle peuvent supporter les chiens et les égratignures?
R. Vous serez heureux d’apprendre amis des animaux que les planchers de vinyle Cali ont été conçus avec une couche d’usure des plus résistantes pour qu’ils conservent leur belle apparence pour des années à venir même avec des amis sur quatre pattes de toutes dimensions.
Q. En quoi Cali Vinyl est différent des autres planchers de vinyle?
R. Conçus pour durer toute une vie – Les planchers de Vinyle Cali sont soigneusement conçus pour exceller dans les environnements les plus exigeants. Peu importe le climat ou le type de pièce dans la maison, les Vinyles Cali ne cesseront de vous impressionner et ce, pour des décennies à venir.
Plus Épais, c’est encore mieux – L’équipe de techniciens a été mis au défi afin qu’ils mettent au point la couche d’usure la plus épaisse et performante possible sur le marché et ils ont livré! Ultra épaisse et développée pour des applications commerciales pour conserver un aspect de plancher fraîchement installé pour des années à venir.
Ne craignez plus l’humidité – Les planchers Cali Vinyle ont été construit pour être 100% à l’épreuve de l’eau. Idéal pour les pièces humides de la maison telles que cuisine, salle de bain et sous-sol. Cali Vinyle élimine les problèmes d’humidité pour de bon.
Le Silence est d’or – La membrane intégrée de liège 100% recyclée de 1.5 mm comprise dans l’épaisseur du 7 mm de la planche réduit les bruit de pas à l’intérieur des pièces et entre les étages de façon efficace et même excède les exigences requises du code du bâtiment pour les habitations.?
Q. Est-ce que les planchers de vinyle sont compatibles avec le système à chaleur radiante?
A. Oui! Ils sont compatibles jusqu’à 29°C (85°F).
Q. Est-ce que l’exposition des planches de vinyle de luxe à la lumière directe UV peut les affecter?
R. Oui! Tout comme pour les autres types de planchers, il sera mieux de limiter la lumière directe sur le plancher en installant des rideaux ou stores aux endroits exposés aux rayons UV élevés.
Q. Puis-je installer des tapis ou carpettes sur mon plancher de vinyle de luxe?
R. Oui! Cependant, éviter les tapis avec endos de caoutchouc ou tissés en matériaux denses : ils empêchent une bonne aération sous le tapis et emprisonnent les saletés abrasives et l’humidité. Il est à noter que le caoutchouc pourrait tacher votre plancher. Nous recommandons plutôt les tapis composés de matériaux perméables à l’air pour mieux gérer l’humidité. Les tapis de mailles ou à motifs de quadrillage sont les meilleurs.
Q. Avec Cali Vinyle, dois-je me soucier du gondolement des bords de planches?
R. Non! Les planchers Cali Vinyle sont fabriqués avec une couche d’usure commerciale de 20mil (7mm d’épaisseur) et un coeur composé de BPC (bamboo composite core) pour tolérer le trafic élevé, les animaux de compagnie et les enfants. Ainsi notre couche d’usure résistante aux égratignures conservera un aspect de plancher fraichement installé pour des années à venir.
Certaines générations de plancher de vinyl précédente était construites extrêmement minces et malléables comme un papier peint mais pour un plancher. Cali Vinyle représente la prochaine génération de plancher de Vinyle de Luxe, des planchers repensés pour une meilleure performance.





 transition requises.
transition requises.
 planchers.
planchers.